what mass of hydrogen is needed to react with carbon monoxide to produce 100000 grams of methanol
![]()
To understand stoichiometry, you'll take to know how to use moles, and you might want to castor upward on your basic chemical reactions. You should accept practiced reaction balancing skills.
What is stoichiometry?
When the space shuttle took off it used two different rocket systems. The main engines were powered past combining oxygen and hydrogen in the highly exothermic reaction
The fuel was stored in that brown tank in liquid grade as liquid oxygen (LOX) and liquid hydrogen (LH2). Liquids are much more dumbo than gases, so storage in the liquid phase allows the shuttle to carry an immense amount of fuel.
Unfortunately, that fuel made up the majority of the weight of the shuttle at liftoff. So nearly of what the shuttle engines lifted from the footing was fuel. That means it was very important not to take too much of one or the other reactant on board. We'd want just enough hydrogen to react with the amount of oxygen on board, and vice versa. That's where stoichiometry and moles are essential.
And maybe yous can be the i to invent a kind of rocket propulsion that volition release more energy per pound of fuel!

Stoichiometry is the human relationship between the relative quantities of substances involved in a chemical reaction, typically ratios of whole numbers. Stoichiometry is a catch all word for all of the calculations we exercise to determine how much of what to mix with what.
Example 1 – Space shuttle fuel mixture
Let's begin with a calculation relevant to our opening example: The space shuttle LOX tank held 629,340 Kg (about 630 metric tons) of LOX. How many kilograms of Hydrogen would it need to comport in order to assure that the reaction
![]()
goes to completion, with no reactants remaining? (The backward rate of this reaction is extremely ho-hum compared to the explosive forward rate, thus the single arrow)

In the calculation above, I've combined the conversion from Kg to grams with the adding of the number of moles of Otwo (32 grand/mol). That's easy if we keep track of units. Now that we have the number of moles of O2, we use the balanced chemical equation (that'southward what information technology's for!) to find out how many moles of H2 we demand. From the coefficients, yous can run into that ii moles of Hii are consumed for every one mole of O2, so nosotros need iii.94 x xvii mol of H2 (1.97 10 2 = 3.94)
The mole ratio told united states of america that in this reaction exactly twice the number of moles of Htwo are used every bit O2. Now it's simply a simple affair of converting 3.94 x tenfour mol of H2 to Kg of H2.
We could move forward and complete the calculation one step at a time - moles O2 to moles H2 to grams H2 to Kg H2, but there'southward a faster way if we make good use of units and cancellation of units. Here's how to combine the 2d function of the adding into one big pace:

Notice that in each of the large fractions above, a relationship and its units are written, and written in such a fashion that the units cancel with those of the previous term to go us closer to the desired units, in this example Kg of H2.
So about 79,000 Kg of Hii would be needed to react with 629,340 Kg of LOX.
Information technology turns out that in practise the shuttles carried a niggling more LH2 than that because of engine efficiency bug and because the rate of loss of lighter LH2 due to evaporation during the await for launch is higher than that of LOX.
Nevertheless, I hope yous get the idea. This is a very of import calculation and information technology depends 100% on doing practiced stoichiometry.
Example 2
How many moles of nitric acrid (HNO3) are required to completely dissolve 1 g of copper (Cu) according to the reaction

At present that we've got the number of moles of copper, the counterbalanced equation (check it!) tells us that for every one mole of Cu, 2 moles of nitric acrid are needed, and then we need 3.64 x 10-2 moles of HNO3. That'due south information technology!
We could, of form, convert the number of moles of HNO3 to grams and and then even to milliliters of HNO3 solution if we know its concentration.
Example 3
In the to a higher place example, how many grams of a 30% (by weight) aqueous solution of HNO3 would exist required to run the reaction to completion?

That's how many grams of HNO3 we need, at present we need to discover out how much of the 30% solution that translates to.The calculation nosotros want is:

Often (especially in the biological sciences) solution concentrations are given in pct, either weight of solute divided by total weight of solution %(w/w) or weight of solute over total book of solution %(west/v).
See the notes on concentration to brush upwards on how to specify the concentration of solutions.
Case 4
n-octane (CviiiH18) is a chief component of gasoline. Information technology oxidizes (burns) in the presence of oxygen according to the equation
If 1 gallon of gasoline is burned in the presence of excess oxygen (i.e. plenty oxygen to brand the reaction go to completion), how many liters of COtwo gas are emitted into the temper? In that location are 3.785 liters in a gallon and the density of gasoline is about 0.75 Kg/liter. At atmospheric temperature and pressure, 1 mole of COii gas takes up about 22.4 liters of book.

Now we can apply the density of gasoline to find the estimate mass of octane (we're bold that gas is 100% octane hither, not also bad an approximation).
Here's the mass of octane:

At present catechumen to moles:

Now we apply the mole ratio in the balanced equation: For every two moles of C8H18 we form 16 moles of CO2.

Now we can use the molar book of CO2 gas (the volume that one mole takes up - given) to discover our result:

In that location are 1000 liters in a cubic meter, and so that's about 4.5 cubic meters of CO2 gas emitted from every gallon of gas burnt. Something to call up about.
Now we could easily have done this whole adding at once by but multiplying the many terms in parenthesis above and canceling units. Here'south how it looks:

All of the units cancel nicely in a nice progression from gallons of octane to liters of COii. Think that division is just multiplication by the reciprocal and that multiplication is commutative, so these numbers can be multiplied and divided on a calculator in whatsoever club, like:
= 3.785 · 750 · 16 · 22.4 / (114 ·2) liters
PS: When you written report gases, yous'll acquire that a mole of whatever gas at standard temperature and pressure, T = 273K and P = i atm, occupies 22.four liters. That was Avogadro's original conjecture.
Case 5 — Limiting reactant
Gilded (Au) metal reacts with chlorine gas (Clii) to course gold (3) chloride, AuCliii.
How many grams of AuCl3 will be formed if one m of golden metal is reacted with 2 yard of Cltwo ?
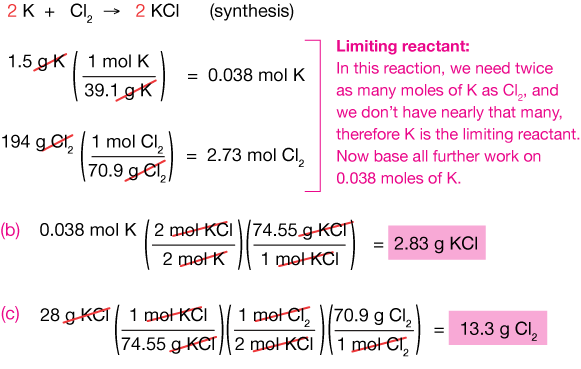
That means that ane of the reactants will run out first, and nosotros phone call this the limiting reactant. When it runs out, the reaction has to stop. So our first job in a problem similar this is to find out which reactant is limiting.
We start by calculating the number of moles of each reactant present:

Now await once more at the reaction:
It says that for every 2 moles of gold, we need 3 moles of chlorine gas. In fact, it's piece of cake to run into that we have more than Cl2 than that. Look at it the other way: The equation says that for every three moles of Cltwo, we need ii moles of gold. It's not even close. Either way we look at information technology, Au is the limiting reactant.
Now that we've identified the limiting reactant, the fox is to base all further calculations on it, because its amount lone determines how far the reaction will proceed. Now information technology's just a matter of finding out how many moles of AuCl3 volition be formed:

(We could accept washed that just past inspection, of course!). Finally, the mass of AuCl3 produced:

Example 6 — Limiting reactant
152 grams of carbon monoxide (CO) are added to 24 g of hydrogen gas (H2) to produce methanol (CH3OH). How many grams of methanol* will be produced?
*
Well, that's an unbalanced reaction, so nosotros ought to balance it. That turns out to exist unproblematic:
At present we merely need to calculate the number of moles of CH3OH nosotros could expect if 24 thousand of H2 reacted completely (bold excess CO) and if 152 thou of CO reacted completely (assuming excess H2). The lesser number of moles will exist the theoretical maximum number of moles of CH3OH formed. First the H2:

So the CO:

At present information technology'due south easy to encounter that the limiting reagent is CO. When 5.4 moles of methanol has been produced, the supply of CO will be exhausted, with 0.6 moles of H2 remaining. Now we merely need to calculate the mass of CHthreeOH that is 5.4 moles:

Solving limiting reactant problems
To solve a limiting reactant trouble, calculate the number of moles of the desired production that would be obtained from each of the reactants, assuming an excess of all other reactants. The reactant that yields the to the lowest degree production is the limiting reactant, and that yield of product is the theoretical maximum yield of the reaction.
Example vii — Ane more than limiting reactant problem ...
93 Kg of nitrogen gas (Northward2) are added to 265.viii Kg of hydrogen gas (H2) to produce ammonia gas (NH3). How much ammonia (in Kg) can be produced by this reaction?
Due northii + iii H2 → 2 NH3
Now we summate the number of moles of NH3 we could expect if 93 Kg of North2 reacted completely (assuming excess Htwo) and if 265.viii Kg of H2 reacted completely (assuming backlog N2). The bottom number of moles will be the theoretical maximum number of moles of NHiii formed. Outset the Northward2:

Then the H2:

Well, it'southward very easy to run across that the limiting reagent is Nii. When 6,643 moles of ammonia has been produced, the supply of Due north2 will exist exhausted, with many more moles of Htwo remaining. Now we just need to calculate the mass of NHiii that constitutes vi,643 moles:

Why is the synthesis of ammonia (NH3) important?

You will recall from your study of bonding that the triple bond in Due north-N is very potent. In fact, there are only a couple of things in nature that tin can break it. One is an energetic electric arc similar in lightning. Another very important one is the biochemistry of plants called legumes. These plants can break the Due north-N bond to form atomic nitrogen thats available for use in making amino acids and nucleic acids. We couldn't go along without them.
In modern agriculture, it'south very important to take non-N2 nitrogen bachelor in the soil, only that supply gets depleted over time as we grow more food on the land, so we need to add together nitrogen-rich fertilizer to it. The principal source of that nitrogen is fertilizer. The reaction to synthesize ammonia is run using a catalyst and the process is chosen the Haber procedure. It'south ane of the most important chemical reactions we take.
Practice problems
| 1. | Consider the reaction Remainder the equation if necessary. If 27.5 g of nitrogen gas (Northward2) are used in the reaction, what mass of fluorine (F2) would be needed for the reaction to get to completion? |
| 2. | Consider the reaction Residue the equation if necessary. If only 18.3 yard of O2 is bachelor, how much C6Hx (cyclohexene) can be reacted? How much CO2 (in grams) would exist produced by this reaction? |
| three. | Consider the reaction
|
| 4. | In 2018, citizens and businesses of the US burned nearly 29.9 trillion cubic anxiety of natural gas. That's roughly 1.half dozen × 109 Kg of propane, if nosotros assume that natural gas is mostly propane (not a terrible supposition, only information technology also contains marsh gas, butane and other small hydrocarbon gases and contaminants).
|
| v. | Consider the reaction:
|
| 6. | Consider the reaction
|
| seven. | Consider the reaction:
|
| 8. | Methyl mercaptan (CH4S or CH3SH) is 1 of the stinky (rotten egg odor) gases added to household natural gas and so that humans tin can detect leaks before existence asphyxiated by them. The combustion of methyl mercaptan produces sulfur dioxide:
|
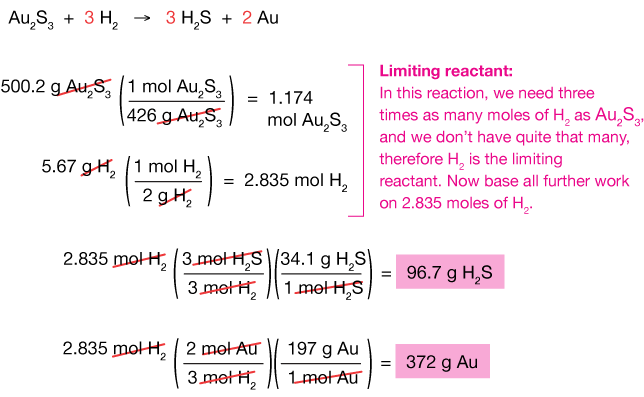
| 9. | Consider the reaction: If 500.2 g of Au2Siii (gold sulfide) and 5.67 chiliad of Hii react, calculate the amount (in grams) of each of the products. |
| 10. | Consider the reaction: If 58.ane g of Mg3Northward2 and 20.4 chiliad of HtwoO react, summate the masses of each of the products. |
Video examples
i. Cu(southward) + AgNO3 (aq) → Cu(NOthree)2 (aq) + Ag(south)
How many grams of copper metallic (assuming excess AgNO3) would it take to form two.eight g of silver metallic, assuming that the reaction proceeded to completion? When you see linguistic communication like "excess AgNOiii" you can presume that this is not a limiting reactant problem.
Minutes of your life: two:59
2. KClOiii (aq) → KCl (aq) + Otwo (yard)
If 19.5 thousand of oxygen (O2) are formed by this reaction, calculate the amount of KClOthree that must have been initially present. Assume that the reaction goes to completion.
Minutes of your life: 2:xx
three. A limiting reactant problem
eight g of gold (Au) react with one.i. liters of Cltwo gas at ii atm. pressure (T = 250˚C) to produce gilt (III) chloride. How much AuCl3 can perchance be produced past this reaction?
In this problem, you'll have to write a balanced reaction first. The number of moles of Cl2 is determined using the ideal gas law: n = PV/RT. Observe that this is a limiting reactant problem because we have fixed amounts of all reactants.
Minutes of your life: v:18
four. AuiiDue south3 (aq) + Hii (g) → HiiS (g) + Au (southward)
If 500.2 thou of Au2South3 and 5.67 m of H2 react, calculate the amount (in grams) of golden metallic that will be formed. Notice that this is a limiting-reactant problem: We know the amounts of both reactants,and it's up to us to determine the maximum amount of product that can be obtained from that mixture.
Minutes of your life: 5:38
![]()
xaktly.com by Dr. Jeff Cruzan is licensed nether a Creative Eatables Attribution-NonCommercial-ShareAlike 3.0 Unported License. © 2012, Jeff Cruzan. All text and images on this website not specifically attributed to some other source were created by me and I reserve all rights as to their apply. Whatever opinions expressed on this website are entirely mine, and do not necessarily reflect the views of any of my employers. Please feel free to send any questions or comments to jeff.cruzan@verizon.net.
Source: https://xaktly.com/ChemistryStoichiometry.html








0 Response to "what mass of hydrogen is needed to react with carbon monoxide to produce 100000 grams of methanol"
Post a Comment